
What Is the Cleavage of a Diamond: Unveiling Its Structural Secrets
When discussing the cleavage of a diamond, I'm referring to the way a diamond can be split or cleaved along specific structural planes due to its atomic structure. As the hardest known natural material on the Mohs scale, a diamond's robustness is often assumed to be uniform in all directions. However, despite its overall strength, a diamond's crystal lattice possesses certain directions along which it is more vulnerable to separation. These planes of weakness follow the paths of the atomic bonds within the crystal structure, allowing for a smoother and more predictable split when properly executed.
Cleavage is a critical concept in both gemology and the diamond cutting process. I understand that not only does it influence how diamonds are cut to maximize their brilliance and value, but it also affects their durability and resistance to breakage. The most common cleavage plane in a diamond is along the octahedral {111} planes. Mastery of cleavage techniques allows diamond cutters to shape these gemstones optimally, conserving as much material as possible while enhancing the stone's optical properties.
My knowledge in this area also tells me that the cleavage characteristics of diamonds have implications beyond jewelry. Understanding these properties is essential for various industrial applications of diamonds, such as in cutting tools, and in high-pressure scientific research, where precision and the ability to manipulate the physical structure of diamond can lead to breakthroughs in material sciences and engineering.
Formation and Extraction
In examining the creation and procurement of diamonds, we focus on two key phases: formation, which is a marvel of nature yielding one of the most sought-after gemstones, and mining and retrieval, a testament to human ingenuity in extracting these gemstones from the depths of the earth.
Formation
| Aspect | Formation | Extraction |
|---|---|---|
| Origin | Naturally occurring under high pressure and temperature in the Earth's mantle. | Extracted from kimberlite or alluvial deposits through mining. |
| Composition | Carbon atoms arranged in a crystalline structure. | Extracted as rough diamond crystals, which undergo cutting and polishing. |
| Formation Process | Occurs over millions of years through geological processes. | Extracted using various mining techniques, such as open-pit or underground mining. |
Diamonds form deep within the Earth's mantle, where carbon atoms are subjected to extreme pressure and temperature conditions. Over billions of years, these atoms bond in a crystal structure, resulting in the hardest minerals known to man. In rare instances, such as through volcanic eruptions or meteorite impacts, these gems are delivered closer to the Earth's surface, embedded within volcanic magma.
- Pressure: Typically over 50 kilobars
- Temperature: Approximately 900 to 1,300 degrees Celsius
- Location: 140 to 190 kilometers below the Earth's surface
Mining and Retrieval
Once potential diamond deposits are located, mining commences, often through open-pit or underground methods. I ascertain the quality and quantity of these deposits through core samples, which guide the extraction process. The process is precise and painstaking, requiring careful handling to ensure the diamond's integrity.
- Open-pit mining: Used for deposits near the surface.
- Underground mining: Deployed when diamonds are deeper underground.
- Retrieval: Ensuring minimal damage to the surrounding structure and the diamonds themselves.
Physical and Chemical Properties

In exploring the physical and chemical properties of a diamond, I am particularly intrigued by its unique chemical composition, impressive physical characteristics, and distinctive optical properties. These facets come together to define a diamond's quality and value.
Chemical Composition
Diamonds are comprised solely of carbon atoms. Each carbon atom forms a strong covalent bond with four other carbon atoms in a tetrahedral lattice. This exceptionally stable structure underpins a diamond's famed hardness and thermal conductivity, allowing it to efficiently dissipate heat. The chemical bond does not easily break, making diamonds incredibly durable and resistant to chemical reactions at standard conditions.
Physical Characteristics
My focus on diamond's physical characteristics leads me to note its unparalleled hardness. Diamonds top the Mohs hardness scale, sitting at 10, which makes them the hardest known natural material. They have a specific gravity of 3.5–3.53. Additionally, the crystal structure provides not only hardness but also a high degree of thermal conductivity, surpassing most other materials.
- Hardness: 10 (Mohs scale)
- Specific Gravity: 3.5–3.53
- Thermal Conductivity: Exceptional
Optical Properties
Brilliance
The ability of a diamond to reflect white light internally and externally, creating sparkle.
Fire
The dispersion of white light into spectral colors, producing flashes of color.
Scintillation
The flashes of light and dark areas as a diamond moves, creating sparkle and liveliness.
Luster
The surface shine or brilliance of a diamond, resulting from light reflection.
A diamond's optical properties are characterized by its refraction, dispersion, and luster. The crystal's ability to bend light, or its refractive index, enhances its brilliance. Its dispersion of light imparts the dazzling colors seen in the "fire" of the diamond. Lastly, the quality of clarity is determined by the absence of internal flaws or inclusions, which can impact the overall luster and brilliance of the diamond.
- Refractive Index: High
- Dispersion: Notable
- Luster: Adamantine
These attributes are integral to understanding a diamond's beauty and structurally impressive nature.
Diamond Structure and Cleavage
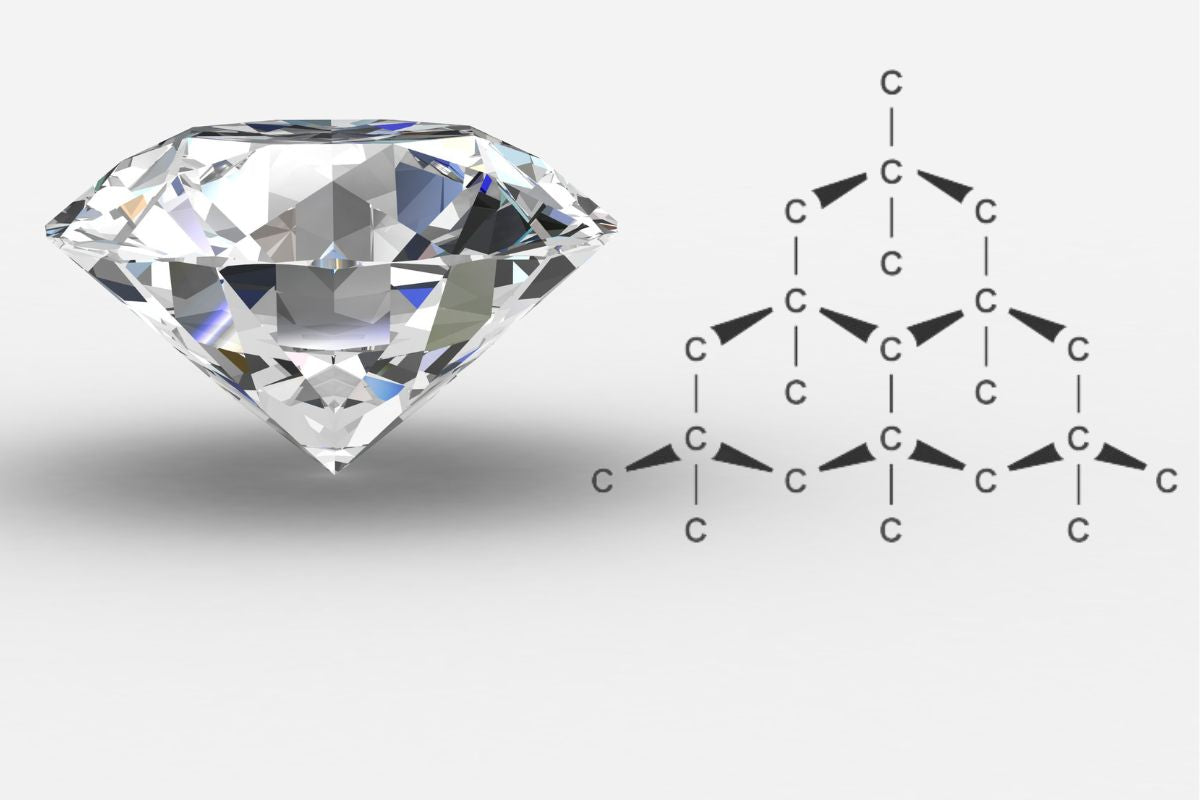
In my examination of diamond structure and cleavage, I focus on how the arrangement of carbon atoms affects the gemstone's cleavage properties.
Crystallography of Diamonds
Diamonds are renowned for their extraordinary hardness, yet they possess planes of structural weakness known as cleavage planes. I understand these planes are a result of diamond's crystalline structure, where carbon atoms are arranged in a repeating pattern called a lattice. Diamonds crystallize in the isometric system, typically forming shapes such as octahedra, cubes, and dodecahedra. Because of the way the carbon atoms are tightly bonded in a three-dimensional network of tetrahedra, diamonds exhibit their well-known hardness.
However, this crystalline arrangement also dictates the possible cleavage directions. A diamond generally has preferred directions of cleavage, with the octahedral {111} planes being the most prominent. When force is applied correctly on these planes, the bonds between atoms can be broken, leading to a clean split.
Understanding Cleavage
| Aspect | Definition | Impact on Gemstone |
|---|---|---|
| Cleavage | The tendency of a gemstone to break along specific planes due to its crystal structure. | Can affect the durability and appearance of the gemstone, influencing its value. |
| Causes | Internal stresses, structural weaknesses, or external factors like impacts or pressure. | May lead to cracks, fractures, or uneven surfaces, affecting the gemstone's quality. |
| Identification | Examined under magnification or with specialized tools to detect cleavage planes. | Helps gemologists assess the gemstone's quality and potential risks of damage. |
Cleavage in gemstones, especially diamonds, is a critical consideration for gem cutters. The cleavage property refers to the tendency of a crystal to break along certain crystallographic planes. In diamonds, octahedral cleavage is paramount. This defines the ability of the crystal to split along the planes of the octahedron, which translates to the {111} planes in crystallography terms.
In contrast, parting is when a crystal separates along a plane of structural weakness that is not a cleavage plane; however, in diamonds, this is less common than true cleavage. By employing precise strikes, gem cutters can exploit these planes to shape the diamond without resorting to more abrasive methods, which preserves the inherent beauty and integrity of the stone.
In practice, diamonds may fracture in ways that do not follow the cleavage planes, particularly if the gemstone contains irregularities. While graphite also consists of carbon atoms, unlike diamond, it forms in a hexagonal system, resulting in softer, more flexible sheets that lack the strength and cleavage properties of diamond.
The mastery of diamond cleavage is essential; a flawless execution can mean the difference between a beautifully faceted gemstone and a fractured piece of carbon. My focus on the cleavage of diamonds embraces the precision and knowledge required to manipulate one of nature's hardest materials.
Diamond Cuts and Crafting

In the meticulous world of gem cutting, the cleavage of a diamond is fundamental to shaping its brilliance. As a gem cutter, I adhere to precision methods to ensure each cut maximizes a diamond's aesthetic and value.
Cutting Techniques
The art of cutting a diamond begins with an understanding of its crystal structure. Diamonds are typically found in shapes like octahedron or cube. I examine these natural formations to determine the diamond's cleavage planes, the areas where a diamond can be split with the least resistance.
For cutting, two primary methods are employed: sawing and cleaving. Sawing is executed with a laser or a thin wire coated with diamond dust, which slices through the diamond. Alternatively, cleaving is applied to larger stones to split them into smaller, more manageable pieces along their natural cleavage lines.
After segmenting the rough diamond, I use specialized tools for polishing and defining the facets, the flat surfaces on a diamond's exterior. The location, size, and angle of each facet are crucial—poorly placed facets can lead to unwanted fractures or a loss of the stone's inherent beauty. Each facet must be polished to a mirror-like finish, allowing the stone to reflect and refract light optimally.
Shapes and Styles
The final appearance of a diamond, both visually and in performance, is significantly influenced by its cut. The desired shape guides the initial design, whether it's the classic round brilliant, princess, or marquise. Once the shape is determined, I carefully create the diamond's pavilion facets, which sit below the girdle (the diamond's widest part), before sculpting the crown facets above.
The GIA (Gemological Institute of America) has established grading standards that I follow meticulously to ensure the diamonds meet specific proportions and symmetry, factors that greatly affect how the diamond interacts with light. The most sought-after diamonds possess a balance between their proportions and polish, which contributes to their brilliance, dispersion, and scintillation.
As I set the diamond into jewelry, the setting process must not only secure the diamond but also complement its cut, allowing light to enter and exit the stone with minimal obstruction. This harmonious union between cut and setting plays a pivotal role in showcasing the diamond's fire and sparkle.
Frequently Asked Questions
In discussing the fascinating world of gemology, I will address some key questions about a diamond's cleavage, which is essential to its handling and value in the industry.
How is cleavage described in diamonds?
Diamond cleavage refers to the way a diamond can split along certain planes of its crystal structure. These cleavage planes are areas of structural weakness where the atomic bonds are less tight, making the diamond more susceptible to splitting along those planes when force is applied.
What terminology is used for diamond cleavage in the gem industry?
The gem industry often describes diamond cleavage in terms of its directionality—most commonly, the octahedral, dodecahedral, or cubic cleavage directions, with octahedral being the most significant and prevalent form.
How does the cleavage of a diamond affect its durability?
The cleavage of a diamond can be a double-edged sword; it's a factor that can influence its susceptibility to splitting or damage when struck. A diamond's durability in the face of blunt force heavily relies on the orientation and quality of these cleavage planes.
Is there a difference between diamond cleavage and fracture?
Yes, there is a difference. While cleavage describes the tendency of a diamond to split along well-defined, flat surfaces parallel to its crystallographic planes, a fracture refers to the breakage of a diamond in a more irregular and less predictable manner, not aligned with the cleavage planes.
What are the implications of cleavage planes on diamond cutting?
Cleavage planes play a pivotal role in diamond cutting. Craftsmen leverage these planes to split diamonds accurately in preparation for further refinement. Understanding and respecting these planes is crucial for achieving the desired shape and optimizing the brilliance of the final cut.
How do cleavage properties vary among different minerals?
Different minerals exhibit varying cleavage characteristics based on their crystal lattice structure. Unlike diamond, which has a distinct cleavage, other minerals may have no cleavage or multiple cleavage directions, influencing their perceived quality and handling in jewelry making.
Checkout some of our top collections:
Leave a comment
Please note, comments must be approved before they are published.










